ISO 9001 Corrective Action Checklist Template
Implementing the ISO 9001 standard is crucial for organizations committed to achieving quality excellence. As part of the ISO 9001 framework, organizations must establish and execute a corrective action process to address nonconformities and improve overall performance. This Corrective Action Checklist for ISO 9001 provides a step-by-step guide to ensure that organizations effectively identify, analyze, and take corrective action to prevent nonconformities from recurring. By following this checklist, organizations can enhance their quality management system and maintain compliance with the ISO 9001 standard.
Understanding The Importance of Corrective Action in ISO 9001
Corrective action is a fundamental aspect of the ISO 9001 standard for quality management. It plays a vital role in addressing nonconformities and improving overall performance within an organization. Understanding the importance of corrective action is crucial for organizations looking to maintain compliance with ISO 9001 and continuously improve their quality management system.
Organizations can identify and resolve issues at their root cause by implementing effective corrective action rather than only treating the symptoms. This proactive approach helps prevent the recurrence of nonconformities, leading to increased customer satisfaction, operational efficiency, and ultimately, quality excellence.
When organizations fully comprehend and appreciate the significance of corrective action, they are better equipped to monitor their processes, identify areas for improvement, and drive continuous growth and improvement. The next section of this checklist will delve deeper into implementing an effective corrective action process in line with ISO 9001 requirements.
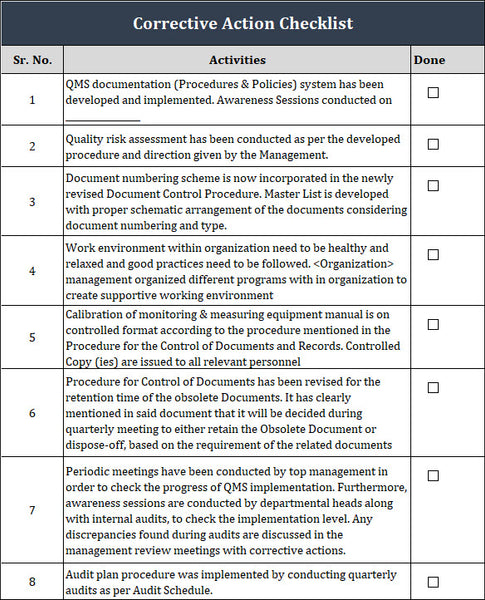
Corrective Action Checklist of ISO 9001
Preparing for corrective action implementation of ISO 9001 involves several steps to ensure the process is effective and efficient. Here is a step-by-step guide to help you prepare for corrective action implementation:
1. Understand the ISO 9001 Requirements: Familiarize yourself with the corrective action requirements stated in ISO 9001. Understand why it is important, what it entails, and how it fits within your quality management system.
2.Train Personnel: Educate and train your personnel on the concepts and principles of corrective action. Ensure they understand the purpose, process, and their roles and responsibilities in executing corrective actions.
3. Establish Clear Procedures: Develop and document procedures for implementing corrective actions. This should include steps to identify, investigate, analyze, and resolve nonconformities or deviations from quality requirements.
4. Identify and Prioritize Nonconformities: Conduct regular audits, inspections, customer feedback analyses, or any other means to identify nonconformities or areas for improvement. Prioritize these based on their potential impact on quality, safety, customer satisfaction, or other critical factors.
5. Document Nonconformities: Document all identified nonconformities thoroughly. Include detailed information such as the nature of the nonconformity, its impact, root cause analysis, and any associated risks.
6. Analyze Root Causes: Conduct a thorough root cause analysis for each nonconformity. Utilize tools and techniques such as the fishbone diagram, 5 Whys, or cause-and-effect analysis to identify the underlying causes or contributing factors.
7. Develop Corrective Action Plans: Based on root cause analysis, develop actionable and feasible corrective action plans. Specify the steps to eliminate or mitigate the root causes and prevent recurrence.
8. Assign Responsibilities: To individuals or teams for implementing corrective actions. Ensure they have the authority, resources, and expertise to execute the plans effectively.
9. Establish Monitoring and Verification Mechanisms: Define measurement criteria, performance indicators, or metrics to monitor the progress and effectiveness of corrective actions. Establish verification methods, such as follow-up audits or inspections, to ensure the implemented actions work as intended.
10. Communicate and Train: Communicate all corrective action plans, progress, and outcomes to relevant stakeholders, including employees, management, customers, and suppliers. Provide appropriate training and awareness programs to ensure everyone understands the importance of corrective actions and how to contribute to their success.
11. Regularly Review and Improve: Continuously review and improve your corrective action process to enhance its efficiency and effectiveness. Analyze the data collected, identify trends, and implement preventive actions to avoid recurring nonconformities.
12. Document and Maintain Records: Document all corrective actions, supporting evidence, and outcomes. Maintain these records to prove your commitment to ISO 9001 compliance and continual improvement.
By following these steps, you can effectively prepare for corrective action implementation within the ISO 9001 framework, ensuring compliance and continuous improvement in your quality management system.
Identifying Non-Conformities and Initiating Corrective Actions
Identifying organizational non-conformities is the first step in implementing an effective corrective action process. This can be done through various methods such as internal audits, customer feedback, or monitoring of key performance indicators.
Once a non-conformity is identified, it is important to initiate corrective actions promptly. This involves conducting a root cause analysis to understand the underlying cause of the non-conformity. By digging deep into the root cause, organizations can develop targeted actions to address the issue and prevent its recurrence effectively.
When initiating corrective actions, involving the relevant stakeholders and assigning responsibilities is essential. Clear communication is crucial to ensure everyone understands the actions they need to take and their respective timelines.
By promptly identifying non-conformities and initiating corrective actions, organizations address immediate issues and lay the foundation to prevent similar problems in the future. The next section of this checklist will discuss the importance of documenting and tracking corrective actions.
Reviewing and Improving Corrective Action Processes
To ensure the effectiveness of corrective action processes, reviewing and improving these processes regularly is crucial. This practice allows organizations to identify any gaps or areas of improvement in their approach to corrective actions.
One way to review the corrective action process is by conducting periodic audits or inspections. These reviews help evaluate the overall effectiveness of the process and identify any weaknesses or bottlenecks. Additionally, organizations can seek feedback from stakeholders to gain insights into their experiences and suggestions for improvement.
Based on the review findings, organizations can make necessary adjustments and improve their corrective action processes. This may involve updating procedures, providing additional employee training, or implementing new tools or technologies to streamline the process.
Conclusion
In conclusion, regularly reviewing and improving corrective action processes is essential for organizations to maintain the effectiveness of their quality management systems. Through periodic audits and inspections, organizations can identify any weaknesses or bottlenecks in their corrective action processes and make the necessary adjustments and improvements. Analyzing the effectiveness of corrective actions is crucial in this continuous improvement cycle. It allows organizations to evaluate the outcomes of their corrective actions and determine if they have successfully addressed non-conformities or if further actions are required.
