MDSAP: Advancing Medical Device Regulatory Compliance Worldwide
In the ever-evolving landscape of healthcare and medical technology, ensuring the safety and effectiveness of medical devices is of paramount importance. To achieve this, regulatory bodies around the world have established stringent standards and requirements for medical device manufacturers. One groundbreaking initiative that aims to streamline and harmonize the regulatory process for medical devices is the Medical Device Single Audit Program (MDSAP). In this blog post, we will explore MDSAP, its significance in the medical device industry, its key components, and the impact it has on global regulatory compliance.
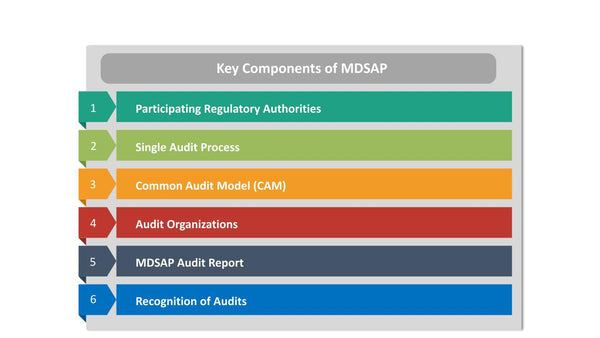
Key Components of MDSAP
To understand the significance of MDSAP, it's essential to explore its key components and how they contribute to achieving regulatory compliance:
- Participating Regulatory Authorities: MDSAP involves a group of participating regulatory authorities, including the U.S. Food and Drug Administration (FDA), Health Canada, the Therapeutic Goods Administration of Australia (TGA), the Ministry of Health, Labor, and Welfare of Japan (MHLW), and the Brazilian Health Regulatory Agency (ANVISA). These authorities work together to oversee the program and ensure its effectiveness.
- Single Audit Process: Under MDSAP, medical device manufacturers undergo a single comprehensive audit of their QMS. This audit covers the requirements of multiple participating regulatory authorities, eliminating the need for separate audits by each authority.
- Common Audit Model (CAM): MDSAP follows a common audit model that outlines the standardized audit processes, procedures, and documentation requirements. The CAM ensures consistency and comparability across audits.
- Audit Organizations: Audits are conducted by organizations known as MDSAP Auditing Organizations (AOs). These AOs are authorized by participating regulatory authorities and adhere to strict criteria for competence and impartiality.
- MDSAP Audit Report: Following the audit, the MDSAP AO prepares a single audit report that details the manufacturer's compliance with the relevant regulatory requirements. This report is then shared with the participating regulatory authorities.
- Recognition of Audits: Each participating regulatory authority recognizes the MDSAP audit report as evidence of compliance with its own regulatory requirements. This recognition streamlines the process of obtaining or maintaining market access in multiple countries.
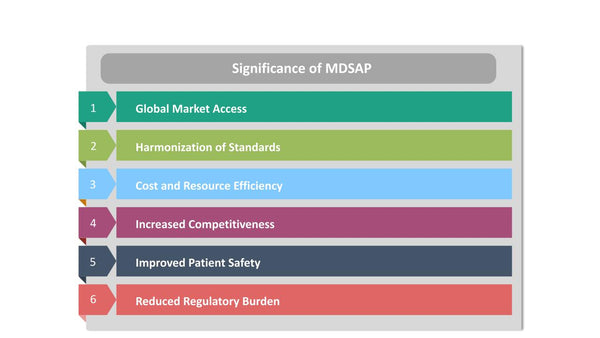
Significance of MDSAP
MDSAP has a significant impact on the medical device industry and regulatory compliance for several reasons:
- Global Market Access: MDSAP facilitates access to multiple international markets through a single audit process. This benefits manufacturers by reducing the time and resources required to navigate different regulatory requirements.
- Harmonization of Standards: MDSAP encourages the harmonization of regulatory standards and requirements among participating authorities. This promotes consistency and clarity for manufacturers.
- Cost and Resource Efficiency: Manufacturers can save time and costs associated with multiple audits, making compliance more accessible, especially for smaller companies.
- Increased Competitiveness: Compliance with MDSAP requirements enhances the competitiveness of medical device manufacturers in the global marketplace. It signals a commitment to high-quality products and regulatory compliance.
- Improved Patient Safety: By promoting adherence to international quality and safety standards, MDSAP contributes to enhanced patient safety and the reliability of medical devices.
- Reduced Regulatory Burden: MDSAP reduces the regulatory burden on both manufacturers and participating authorities, allowing resources to be allocated more effectively.
MDSAP Auditing Process
Understanding the auditing process is crucial for medical device manufacturers seeking to comply with MDSAP requirements. Here are the key steps in the MDSAP auditing process:
- Preparation: Manufacturers prepare for the MDSAP audit by ensuring their QMS is fully compliant with the relevant regulatory requirements. This may involve conducting internal audits and addressing any non-conformities.
- Selection of AO: Manufacturers select an MDSAP Auditing Organization (AO) from a list of authorized AOs. The AO will conduct the audit and issue the audit report.
- Audit: The chosen AO conducts the audit, which includes a thorough assessment of the manufacturer's QMS and associated processes. The audit covers the requirements of participating regulatory authorities, including the FDA, Health Canada, TGA, MHLW, and ANVISA.
- Audit Report: Following the audit, the AO prepares an MDSAP audit report, which details the findings, including any non-conformities or observations.
- Corrective Actions: Manufacturers are required to address any identified non-conformities or observations and provide evidence of corrective actions taken.
- Submission to Regulatory Authorities: The AO submits the audit report and supporting documentation to the participating regulatory authorities. Each authority reviews the audit report to determine compliance with its specific requirements.
- Market Access: Once the participating regulatory authorities accept the audit report, manufacturers can use it as evidence of regulatory compliance to gain or maintain market access in the respective countries.
Benefits and Challenges of MDSAP
MDSAP offers numerous benefits for both manufacturers and regulatory authorities, but it also presents some challenges:
Benefits:
- Streamlined Auditing: Manufacturers benefit from reduced audit burdens and associated costs, as a single audit covers multiple regulatory requirements.
- Global Market Access: MDSAP facilitates entry into multiple international markets, increasing opportunities for manufacturers.
- Harmonized Standards: The program promotes the alignment of regulatory standards, making compliance more straightforward and consistent.
- Enhanced Quality: Manufacturers are incentivized to maintain high-quality standards to meet MDSAP requirements, leading to safer and more reliable medical devices.
Challenges:
- Transition Period: Manufacturers may need time to transition to MDSAP requirements, especially if they were previously compliant with different regulatory standards.
- Cost of Compliance: While MDSAP can ultimately reduce costs, there may be initial expenses associated with aligning with the program's requirements.
- Resource Intensive: Maintaining compliance with MDSAP requires ongoing effort and resources, particularly for larger organizations with complex QMS.
Conclusion
The Medical Device Single Audit Program (MDSAP) represents a significant step towards simplifying and standardizing the regulatory landscape for medical device manufacturers worldwide. By streamlining the auditing process and promoting the harmonization of standards, MDSAP enhances market access opportunities, ensures compliance with stringent regulatory requirements, and ultimately contributes to the safety and quality of medical devices. As the global healthcare industry continues to evolve, MDSAP plays a pivotal role in supporting innovation while safeguarding patient safety and well-being.

